CU-Med Biobank
CU-Med Biobank is a unit operating under the Faculty of Medicine of The Chinese University of Hong Kong. It was established in the Prince of Wales Hospital in 2019. The primary objective of CU-Med Biobank is to acquire, process, store and distribute donors’ biological samples in a standardised and systematic manner. CU-Med Biobank is equipped with ultra-low temperature freezers with capacity more than 1,000,000 vials. All of them are monitored in a 24/7 manner to ensure sample quality.
In April 2023, CU-Med Biobank was officially awarded the ISO 20387:2018(en) accreditation by the American Association for Laboratory Accreditation (A2LA), recognizing its compliance with the international standard for biobanking. This accreditation demonstrates the commitment to maintain the highest quality standards in handling and managing patient biological samples.
In the future, CU-Med Biobank will make every effort to provide valuable samples to researchers of highest quality, contributing to the development of Hong Kong as an important biomedical research hub.

Governance Structure
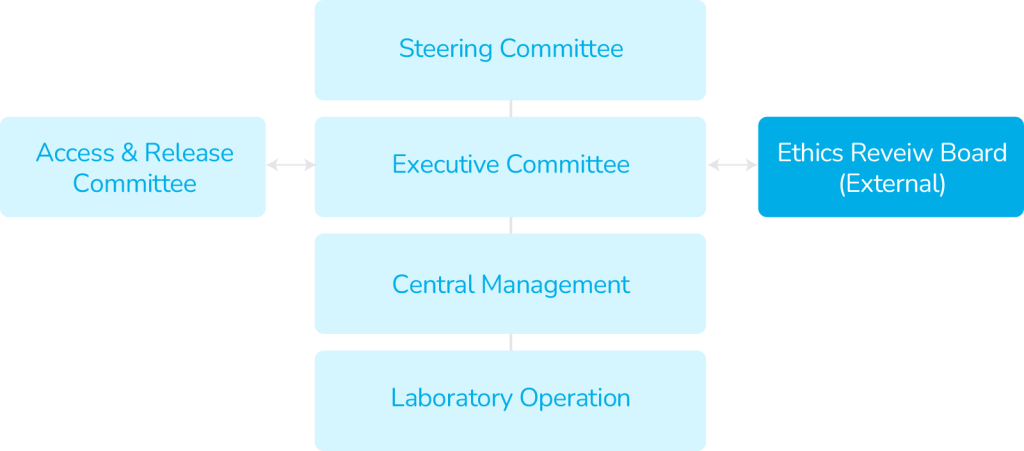
Chairperson of Steering Committee

Prof. Philip WY CHIU
Dean
Faculty of Medicine, CUHK
Executive Committee Members

Prof. Ronald CW MA (Chairperson)
Director of CU-Med Biobank
S.H. Ho Professor of Diabetes
Department of Medicine & Therapeutics
Faculty of Medicine

Prof. Ka Fai TO (Co-chairperson)
Co-Director of CU-Med Biobank
Professor
Department of A & C Pathology
Faculty of Medicine

Prof. James YW LAU
Yao Ling Sun Professor of Surgery
Department of Surgery
Faculty of Medicine

Prof. Brigette BY MA
Frances Lee Professor of Precision Oncology
Department of Clinical Oncology
Faculty of Medicine

Prof. Vincent WS WONG
Mok Hing Yiu Professor of Medicine
Department of Medicine & Therapeutics
Faculty of Medicine

Ms. Nowell CH WONG
Faculty Secretary & Director of Planning
Faculty and Planning Office
Faculty of Medicine
Access & Release Committee Members

Prof. Ronald CW MA (Chairperson)
Director of CU-Med Biobank
S.H. Ho Professor of Diabetes
Department of Medicine & Therapeutics
Faculty of Medicine

Prof. Ka Fai TO (Co-chairperson)
Co-Director of CU-Med Biobank
Professor
Department of A & C Pathology
Faculty of Medicine

Prof. Brigette BY MA
Frances Lee Professor of Precision Oncology
Department of Clinical Oncology
Faculty of Medicine
Central Management and Operation Team

Dr. Terry CT OR
Scientific Officer (Laboratory Development Manager)
CU-Med Biobank, Faculty of Medicine
Email: terryor@cuhk.edu.hk

Miss Fion CY CHUANG
Research Associate
CU-Med Biobank, Faculty of Medicine
Email: fionchuang@cuhk.edu.hk

Mr. Lucius LT LO
Research Assistant
Department of A & C Pathology
Faculty of Medicine
Email: luciuslo@cuhk.edu.hk

Miss Kelly YW CHEUNG
Research Assistant
CU-Med Biobank, Faculty of Medicine
Email: kellyywcheung@cuhk.edu.hk

Miss Denise HY SIU
Research Assistant
CU-Med Biobank, Faculty of Medicine
Email: denisesiu@cuhk.edu.hk

Mr. Ken MF YEUNG
Research Assistant
CU-Med Biobank, Faculty of Medicine
Email: manfaiyeung@cuhk.edu.hk

Miss Cynthia SW LAU
Research Assistant
CU-Med Biobank, Faculty of Medicine
Email: cynthiaswlau@cuhk.edu.hk
Acknowledgement
We would like to extend our sincere gratitude to the Faculty and Planning Office (Finance and Development Team, Information Technology Management Team, Media and Communications Team, Human Resources and Research Management Team) and the Department of Anatomical and Cellular Pathology for their unwavering administrative and technical support to our work at CU-Med Biobank.

Storage Facility
To ensure the utmost security, these valuable samples are stored in a secure facility. Access is strictly restricted to authorized CU-Med Biobank personnel with specialized training and credentials. The storage facility is continuously monitored by a comprehensive 24/7 CCTV surveillance system. Furthermore, each freezer and liquid nitrogen storage is integrated with a temperature monitoring alarm system. In the event of any deviation from the setpoint temperature, CU-Med Biobank personnel will be promptly notified by the system, enabling swift action to maintain sample integrity. These measures altogether ensure the utmost protection of the stored samples.
Data Security
In addition to biological samples, CU-Med Biobank stores personal and clinical information. To ensure proper management, all this information is systematically recorded and maintained in a laboratory information management system (LIMS). The LIMS is hosted on a dedicated virtual machine located within faculty’s secure server environment, fortified by the CUHK and Faculty of Medicine firewall. Access to the LIMS is strictly restricted to authorized CU-Med Biobank personnel, who undergo rigorous training and adhere to established protocols.
To safeguard donor privacy, all personally identifiable information is removed prior to releasing samples and associated data, ensuring complete confidentiality and anonymity.
Sample Acquisition and Processing
CU-Med Biobank undertakes the acquisition and processing of a wide range of sample types, encompassing blood, stool, tissues, urine, oral rinse, breast milk, cerebrospinal fluid, swabs, hair, and nails. Stringent standard operating procedures are followed to ensure meticulous processing of these samples. Sample information is also well documented.
Moreover, quality controls are implemented to ensure the integrity and reliability of the samples within the biobank. By adhering to these stringent protocols, CU-Med Biobank maintains the highest standards of sample quality and data integrity, facilitating valuable research and advancing scientific understanding.
Disclaimer: CU-Med Biobank is only responsible for acquisition of the biological materials. All collection procedures are carried out by collaborating sample depositors.

Accreditation and Certification
In 2019, CU-Med Biobank attained certification from the Canadian Tissue Repository Network (CTRNet), highlighting its adherence to rigorous standards and best practices in acquisition, processing, storage, and distribution of biological samples.
After a review process lasting over four months by the American Association for Laboratory Accreditation (A2LA), CU-Med Biobank was officially awarded the ISO 20387:2018(en) accreditation in 2023, which is an endorsement of its highest standards in biobanking. The accreditation encompasses four key areas: sample acquisition, processing, storage, and distribution. This solidifies CU-Med Biobank’s reputation as a trusted repository for valuable biological samples and demonstrates its commitment to maintaining sample integrity and donor privacy. This will help promote large-scale international research cooperation, attract local, mainland and overseas scientific research teams or pharmaceutical research institutions to collaborate, and thus enhance the efficiency and level of biomedical research.

Publications by Collaborating Research Teams
Su, Q., Lau, R. I., Liu, Q., Li, M. K. T., Yan Mak, J. W., Lu, W., Lau, I. S. F., Lau, L. H. S., Yeung, G. T. Y., Cheung, C. P., Tang, W., Liu, C., Ching, J. Y. L., Cheong, P. K., Chan, F. K. L., & Ng, S. C. (2024). The gut microbiome associates with phenotypic manifestations of post-acute COVID-19 syndrome. Cell host & microbe, 32(5), 651–660.e4.
https://doi.org/10.1016/j.chom.2024.04.005
Peng, Y., Zhu, J., Wang, S., Liu, Y., Liu, X., DeLeon, O., Zhu, W., Xu, Z., Zhang, X., Zhao, S., Liang, S., Li, H., Ho, B., Ching, J. Y., Cheung, C. P., Leung, T. F., Tam, W. H., Leung, T. Y., Chang, E. B., Chan, F. K. L., … Tun, H. M. (2024). A metagenome-assembled genome inventory for children reveals early-life gut bacteriome and virome dynamics. Cell host & microbe, 32(12), 2212–2230.e8.
https://doi.org/10.1016/j.chom.2024.10.017
Zheng, J., Sun, Q., Zhang, M., Liu, C., Su, Q., Zhang, L., Xu, Z., Lu, W., Ching, J., Tang, W., Cheung, C. P., Hamilton, A. L., Wilson O’Brien, A. L., Wei, S. C., Bernstein, C. N., Rubin, D. T., Chang, E. B., Morrison, M., Kamm, M. A., Chan, F. K. L., … Ng, S. C. (2024). Noninvasive, microbiome-based diagnosis of inflammatory bowel disease. Nature medicine, 30(12), 3555–3567.
https://doi.org/10.1038/s41591-024-03280-4
Wang, S., Liu, Y., Tam, W. H., Ching, J. Y. L., Xu, W., Yan, S., Qin, B., Lin, L., Peng, Y., Zhu, J., Cheung, C. P., Ip, K. L., Wong, Y. M., Cheong, P. K., Yeung, Y. L., Kan, W. H. B., Leung, T. F., Leung, T. Y., Chang, E. B., Rubin, D. T., … Zhang, L. (2024). Maternal gestational diabetes mellitus associates with altered gut microbiome composition and head circumference abnormalities in male offspring. Cell host & microbe, S1931-3128(24)00223-3. Advance online publication.
https://doi.org/10.1016/j.chom.2024.06.005
Lau, R. I., Su, Q., Lau, I. S. F., Ching, J. Y. L., Wong, M. C. S., Lau, L. H. S., Tun, H. M., Mok, C. K. P., Chau, S. W. H., Tse, Y. K., Cheung, C. P., Li, M. K. T., Yeung, G. T. Y., Cheong, P. K., Chan, F. K. L., & Ng, S. C. (2024). A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial. The Lancet. Infectious diseases, 24(3), 256–265.
https://doi.org/10.1016/S1473-3099(23)00685-0
Wong, M. C. S., Zhang, L., Ching, J. Y. L., Mak, J. W. Y., Huang, J., Wang, S., Mok, C. K. P., Wong, A., Chiu, O. L., Fung, Y. T., Cheong, P. K., Tun, H. M., Ng, S. C., & Chan, F. K. L. (2023). Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study). Nutrients, 15(8), 1982.
https://doi.org/10.3390/nu15081982
Su, Q., Liu, Q., Lau, R. I., Zhang, J., Xu, Z., Yeoh, Y. K., Leung, T. W. H., Tang, W., Zhang, L., Liang, J. Q. Y., Yau, Y. K., Zheng, J., Liu, C., Zhang, M., Cheung, C. P., Ching, J. Y. L., Tun, H. M., Yu, J., Chan, F. K. L., & Ng, S. C. (2022). Faecal microbiome-based machine learning for multi-class disease diagnosis. Nature communications, 13(1), 6818.
https://doi.org/10.1038/s41467-022-34405-3


